Component Characterization
Understanding Funadamental Interactions in Fuel-Cell Catalyst Layer Inks
 Hydrogen fuel cells are promising energy conversion devices, but there is a critical lack of understanding of the electrode formation process. The electrodes (or catalyst layers, CLs) are heterogeneous porous electrodes comprised of catalyst particles and an ion-conductive polymer (ionomer) that also serves as a binder. The CLs are made from inks (colloidal dispersions of the particles, ionomer, and solvent) and then cast onto a substrate to form the dried electrode. The electrochemical and morphological properties of the CL are influenced by both the processing conditions during the ink deposition process as well as the solution-phase properties of the ink. Despite this, the properties of the ink and how
Hydrogen fuel cells are promising energy conversion devices, but there is a critical lack of understanding of the electrode formation process. The electrodes (or catalyst layers, CLs) are heterogeneous porous electrodes comprised of catalyst particles and an ion-conductive polymer (ionomer) that also serves as a binder. The CLs are made from inks (colloidal dispersions of the particles, ionomer, and solvent) and then cast onto a substrate to form the dried electrode. The electrochemical and morphological properties of the CL are influenced by both the processing conditions during the ink deposition process as well as the solution-phase properties of the ink. Despite this, the properties of the ink and how 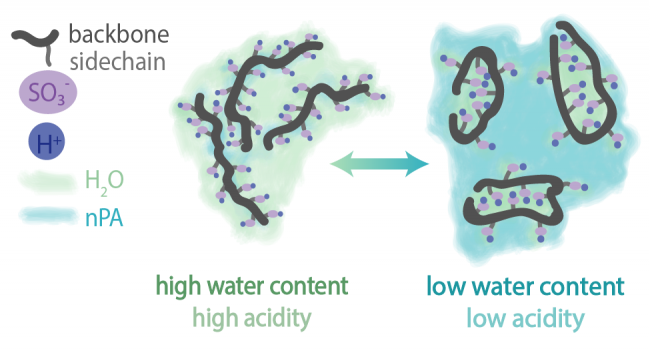 they influence the final properties of the catalyst layer are currently unkown. This project, therefore, seeks to understand the relavent fundamental interactions within the ink (including polymer/solvent, polymer/particle, particle/particle, and particle/solvent) and how controlling these impacts the CL. Recent work has focused on elucidating the effect of solvent on ionomer conformation, binding, and ink aggregation phenomena using a combination of dynamic light scattering, zeta potential and pH measurements, quartz crystal microbalance, and rheology.
they influence the final properties of the catalyst layer are currently unkown. This project, therefore, seeks to understand the relavent fundamental interactions within the ink (including polymer/solvent, polymer/particle, particle/particle, and particle/solvent) and how controlling these impacts the CL. Recent work has focused on elucidating the effect of solvent on ionomer conformation, binding, and ink aggregation phenomena using a combination of dynamic light scattering, zeta potential and pH measurements, quartz crystal microbalance, and rheology.
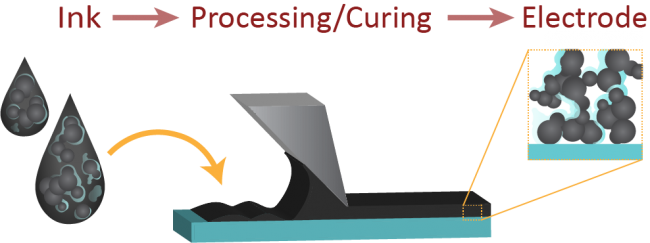
Figure at right: (Top) Schematic describing the CL formation process, going from ink to electrode. Typical casting procedures include ultra-sonic spray coating, doctor blading, and screen printing. (Bottom) Perfluorosulfonic acid is the state of the art ionomer used in hydrogen fuel cells, and consists of a hydrophobic backbone with pendant side chains that terminate on sulfonic acid groups. Due to competing relative hydrophilicity, the ionomer has different conformations in solution as solvent type is changed, including moving from high to low ratios of water to propanol (as typically used in an ink). This impacts the relative acidity of the ink, for the same given ionomer content.
Recent Publications:
Berlinger, Sarah A, Bryan D McCloskey, and Adam Z Weber. "Inherent Acidity of Perfluorosulfonic Acid Ionomer Dispersions and Implications for Ink Aggregation." The Journal of Physical Chemistry B 122.31 (2018) 7790 - 7796
Hatzell, Kelsey B, Marm B Dixit, Sarah A Berlinger, and Adam Z Weber. "Understanding inks for porous-electrode formation." J. Mater. Chem. A 5.39 (2017) 20527 - 20533.
Ionomers for Electrochemical Devices
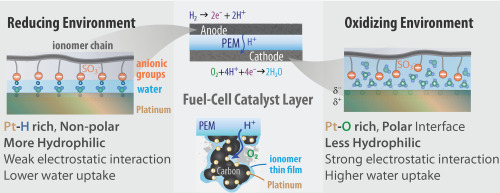 Ion-conducting polymers (Ionomers) are a central component to fuel cells and similar electrochemical energy-conversion devices. Ionomers exist both as a bulk membrane, transporting protons from cathode to anode and separating reactants and products, as well as a thin film in the catalyst layers where they transport protons and gaseous species to reaction sites. To optimize and understand their behavior, we measure their transport, swelling, and uptake properties across the lengthscales to feed into the modeling effort and develop structure-function relationships. Recent work has explored the effect of ionomer thin film structure under oxidizing and reducing environments.
Ion-conducting polymers (Ionomers) are a central component to fuel cells and similar electrochemical energy-conversion devices. Ionomers exist both as a bulk membrane, transporting protons from cathode to anode and separating reactants and products, as well as a thin film in the catalyst layers where they transport protons and gaseous species to reaction sites. To optimize and understand their behavior, we measure their transport, swelling, and uptake properties across the lengthscales to feed into the modeling effort and develop structure-function relationships. Recent work has explored the effect of ionomer thin film structure under oxidizing and reducing environments.
Recent Publications:
Tesfaye, Meron, Andrew N MacDonald, Peter J Dudenas, Ahmet Kusoglu, and Adam Z Weber. "Exploring substrate/ionomer interaction under oxidizing and reducing environments." Electrochemistry Communications 87 (2018) 86 - 90.
Tesfaye, Meron, Douglas I. Kushner, Bryan D. McCloskey, Adam Z. Weber, and Ahmet Kusoglu. "Thermal Transitions in Ionomer Thin-FIlms." ACS Macro Letters 2018 7 (10), 1237-1242