Solar Fuels
Solar Water-Splitting Devices
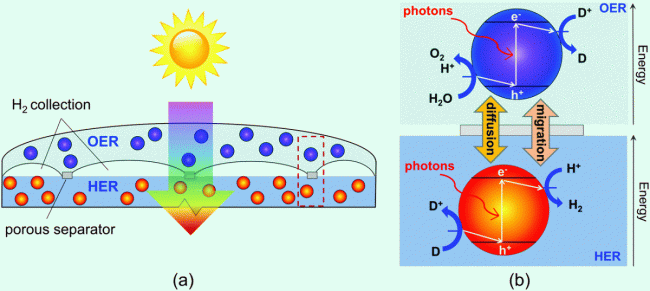 Solar water-splitting is a promising approach to convert and store solar energy in the form of stable chemical bonds. For solar-hydrogen production, particle-suspension reactor designs comprising suspended photocatalysts in an aqueous electrolyte are projected to be cost-efficient alternatives to the more exhaustively optimized fixed-electrode device architectures. We use multiphysics modeling to study device-scale transport, kinetic, and optical processes, and their impacts on the solar-to-hydrogen efficiencies for these designs.
Solar water-splitting is a promising approach to convert and store solar energy in the form of stable chemical bonds. For solar-hydrogen production, particle-suspension reactor designs comprising suspended photocatalysts in an aqueous electrolyte are projected to be cost-efficient alternatives to the more exhaustively optimized fixed-electrode device architectures. We use multiphysics modeling to study device-scale transport, kinetic, and optical processes, and their impacts on the solar-to-hydrogen efficiencies for these designs.
Figure at right: (a) Schematic of the vertically stacked particle-suspension reactor design which affords tandem light absorption for Z-scheme solar water splitting, and (b) the desired chemistry and direction of electron transfer reactions on the surface of the semiconductor particles, assuming acidic conditions.
Recent Publications:
Chandran, Rohini Bala, Sasuke Breen, Yuanxun Shao, Shane Ardo, and Adam Z Weber. "Evaluating particle-suspension reactor designs for Z-scheme solar water splitting via transport and kinetic modeling." Energy & Environmental Science 11.1 (2018) 115 - 135.