Electrolyzers
Water Electrolyzer Optimization
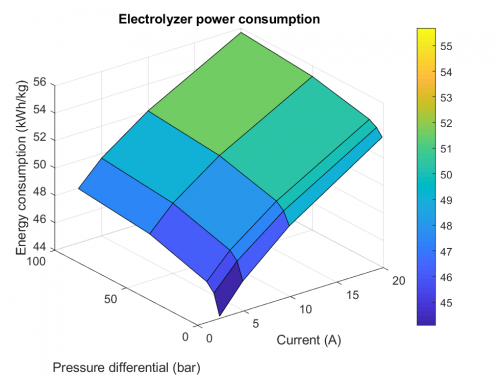 Understanding multiphase transport in proton-exchange-membrane water electrolyzer (PEMWE) is critical to improving electrolyzer performance and optimizing the material. Mathematical modeling is ideally suited to tackle such issues, especially due to the inherent divergent time and lengthscales involved during operation. In a PEMWE, the multiphase transport mainly involves: a) Liquid water transport in the porous electrode; b) Interaction between liquid water and product gas; and c) Gas transport, i.e., removing product gas in the liquid water through the porous transport layer (PTL) and into the channel. For a), as liquid water is the reactant, its distribution in the PTL and catalyst layer is of great importance to the reaction distribution. For b), the gas bubble formation on the electrochemically active surface area (ECSA) blocks the water access and leads to a dynamic redistribution of current, which affects the local and perhaps global cell performance and may lead to enhanced degradation. Lastly, understanding the mechanism of gas bubble detachment is necessary in order to minimize the loss of ECSA due to gas bubble coverage.
Understanding multiphase transport in proton-exchange-membrane water electrolyzer (PEMWE) is critical to improving electrolyzer performance and optimizing the material. Mathematical modeling is ideally suited to tackle such issues, especially due to the inherent divergent time and lengthscales involved during operation. In a PEMWE, the multiphase transport mainly involves: a) Liquid water transport in the porous electrode; b) Interaction between liquid water and product gas; and c) Gas transport, i.e., removing product gas in the liquid water through the porous transport layer (PTL) and into the channel. For a), as liquid water is the reactant, its distribution in the PTL and catalyst layer is of great importance to the reaction distribution. For b), the gas bubble formation on the electrochemically active surface area (ECSA) blocks the water access and leads to a dynamic redistribution of current, which affects the local and perhaps global cell performance and may lead to enhanced degradation. Lastly, understanding the mechanism of gas bubble detachment is necessary in order to minimize the loss of ECSA due to gas bubble coverage.
Anion-Exchange Membrane Electrolysis
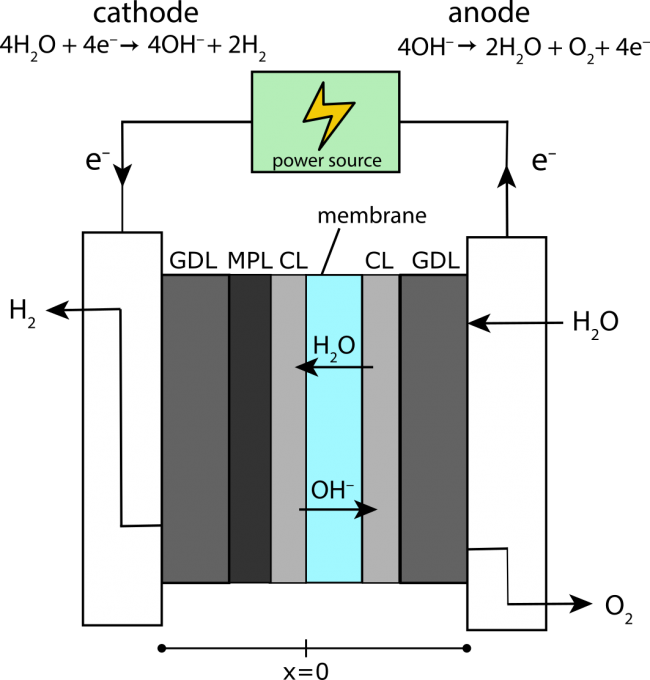
Anion-exchange membrane (AEM) electrolysis is a promising method of producing hydrogen gas from water using electricity. Using an anion-exchange membrane, rather than a porous separator as used in commercial alkaline electrolysis, hydrogen can be produced at high pressure, which enables easier storage and refueling of cars. Additionally, using an anion-exchange membrane rather than a proton-exchange membrane (PEM) allows the use of less expensive catalyst materials.
The performance of AEM electrolyzers, however, has not reached that of PEM or alkaline electrolyzers. In our group, we have developed a model of an AEM electrolyzer to explore ways to improve electrolyzer performance, such as through improved cell design, or the use of electrolyte salts to improve the conductivity of the feed water. Our work could help bring down the cost of hydrogen gas, making an inexpensive, environmentally friendly fuel available for everyone.